We are hearing much about vaccines and antibodies in the COVID era. Could a Lyme antibody treatment be our new hope in the fight against the disease?
A Lyme disease vaccine was introduced in 1998, but was fraught with controversy. Though some folks, who received the FDA-approved Lyme vaccine, reported arthritis post-shot, evidence was not strong enough for the vaccine to be pulled from the market. But eventually, makers of the vaccine discontinued the shot due to lack of sales. Experts have weighed in over the years since, noting a few reasons for the vaccine’s demise. First, the public lost faith in the safety of the shot. There is also the fact that Lyme has a low morbidity rate, which resulted in the public not deeming the infection a true threat. Today, the CDC estimates that there are as many as 300,000 cases of Lyme disease in the United States each year. Though, only about 30,000 are confirmed. Now that chronic Lyme disease is more widely-accepted by the medical community, efforts to prevent infection are expanding.
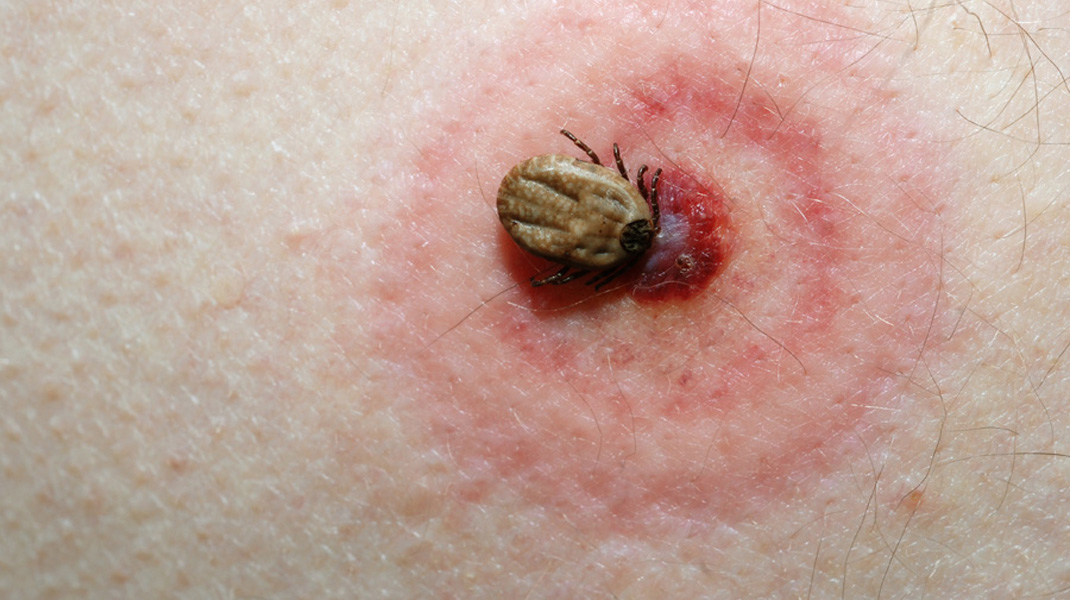
Enter, Phase 1 Clinical Trials for a New Lyme Antibody Treatment
Some scientists spend most of their careers researching and developing inoculations against many types of diseases. One that has been at the forefront over the last decade, is Lyme disease. One group of scientists has gotten the green light from the FDA to begin phase one clinical trials on a Lyme antibody treatment. Their animal trials resulted in an efficacy rate of 100%. The antibody, known as Lyme PrEP, deploys, “a single human antibody, or blood protein, to kill the bacteria in the tick’s gut while the tick drinks its victim’s blood, before the bacteria can get into the human host.” The use of a single antibody reduces the likelihood of unwanted side effects, unlike a vaccine, which triggers the development of many antibodies. Developers of Lyme PrEP note that protection will come from a recurring yearly shot. The goal is to maintain protection against Lyme disease for a full nine-month period. Early trials indicate that this goal will be met, though this will be confirmed by later studies. They hope to have concluded those studies in 2022, and bring the antibody treatment to market in 2023 or 2024.
We must stay the course with current methods of Lyme prevention.
 Until we have a safe and surefire method of Lyme infection prevention, we must employ the tools we have available. The best way to prevent Lyme disease is to lower our risk of encountering ticks. We must wear protective clothing while hiking and camping. Massachusetts residents should choose professional tick control around their property from April through October – and even extend those efforts with tick tubes in the winter. We must perform a tick check on ourselves, our children, and our pets after spending time outdoors. And if you are feeling ill after a known tick bite, seek immediate medical attention.
Until we have a safe and surefire method of Lyme infection prevention, we must employ the tools we have available. The best way to prevent Lyme disease is to lower our risk of encountering ticks. We must wear protective clothing while hiking and camping. Massachusetts residents should choose professional tick control around their property from April through October – and even extend those efforts with tick tubes in the winter. We must perform a tick check on ourselves, our children, and our pets after spending time outdoors. And if you are feeling ill after a known tick bite, seek immediate medical attention.
